Section 5: Chemical Reactions – Rates, Type, and Energy
Many types of chemical reactions can be grouped by their similar reactions. In a synthesis reaction, two or more substances combine to form another substance. An example of this can be seen when oxygen is combined with iron in the presence of water to form hydrated iron (II) oxide or rust.

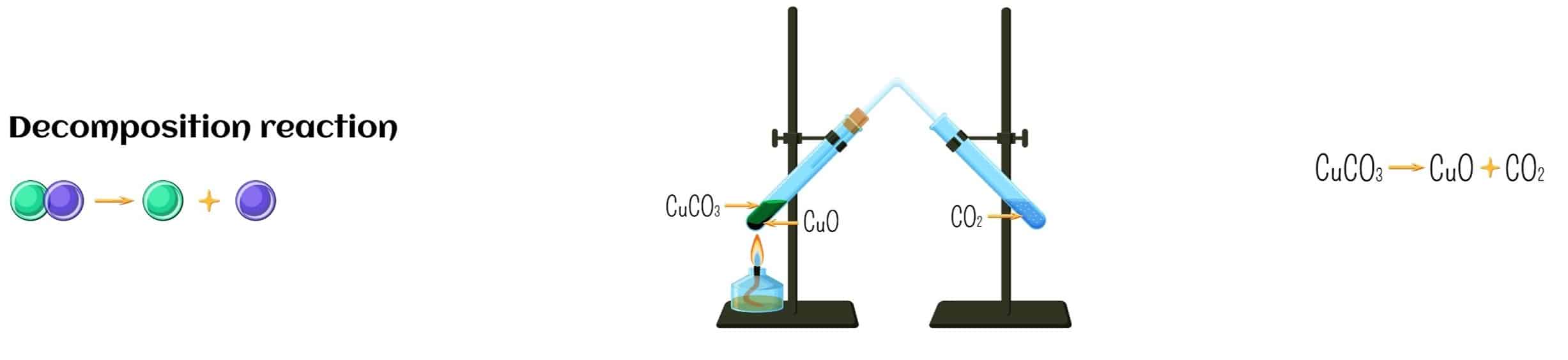
In a decomposition reaction, the reverse happens. One substance breaks down or decomposes into two or more simpler substances. Often this type of reaction requires heat, light, or electricity.
A single-displacement reaction is a reaction in which one element replaces another in a compound. 
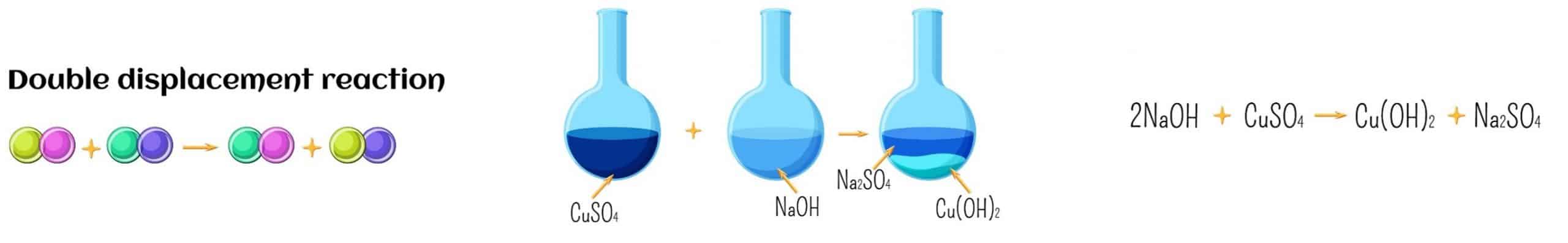
Finally, a double-displacement reaction results in a precipitate, water, or gas when one compound’s positive ion swaps with another compound’s positive ion.
Chemical reactions involve an exchange of energy. An exothermic reaction is when energy is released in the form of heat, as seen in burning wood or exploding fireworks. An endothermic reaction is where heat energy is absorbed, such as photosynthesis. A catalyst speeds up a chemical reaction without itself being permanently changed. An inhibitor prevents or slows a chemical reaction or interferes with the catalyst.
Review:
- What happens during a synthesis reaction?
- What happens in a single-displacement reaction?
- Compare an exothermic reaction to an endothermic reaction.
