Section 2: Types of Mixtures
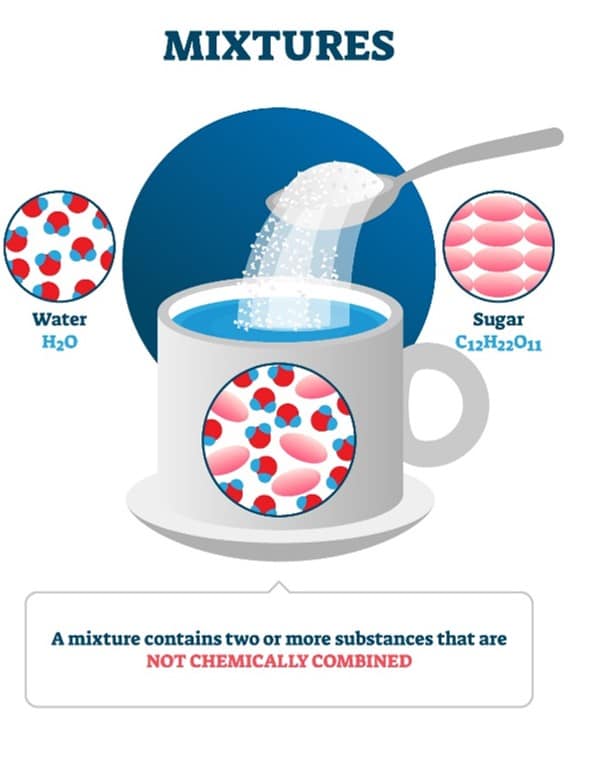
A solution is the most common type of homogeneous mixture comprised of small particles that cannot even be seen with a microscope. A solution must have one or more substances dissolved into another substance. A solution will also never settle at the bottom of its container. Vinegar and hydrogen peroxide are examples of solutions.
A colloid is a mixture of particles larger than the solution but too light to settle. Fog is an example of a colloid. Fog has tiny drops of water spread throughout the air. Detecting colloids can be difficult, but shining a beam of light at a colloid will make the light scatter. This scattering of light is called the Tyndall effect. A suspension is a heterogeneous mixture containing a liquid where visible particles settle. For example, Italian dressing and chocolate milk are suspensions.
Review:
- Identify two characteristics of solutions.
- What is a colloid?
- Explain the Tyndall effect.
