Section 6: Fluids: Behaviors of Liquids and Gases
When you place an object in water, it will either float or sink. Where the object ends up depends on the weight and buoyant force exerted on it. Buoyancy is the ability of a fluid, or any gas or liquid that flows, to exert an upward force on an object immersed in it. This is called the buoyant force. Buoyancy and pressure are related in that the pressure increases with depth.

When scientists began studying gases, they found that all gases behaved similarly when certain conditions were changed. A Greek mathematician named Archimedes discovered that the buoyant force on an object in a fluid equals the weight of the fluid displaced by the object. So, if the fluid is equal to the object’s weight, it floats. If the buoyant force of the fluid is less than the object’s weight, then the object will sink. For example, a boat displaces enough water to equal the weight of the boat; therefore, it floats. This became known as Archimedes’ principle.
When underwater, you can feel the pressure or force exerted by the water around you. A French scientist named Blaise Pascal developed Pascal’s principle, which states that the pressure applied to a fluid is transmitted unchanged throughout the fluid. This can be seen in squeezing the end of a tube of toothpaste. The pressure was transmitted through the fluid toothpaste when squeezed.
Swiss scientist Daniel Bernoulli studied the properties of moving fluids through air and water. He observed a relationship between the velocity of a fluid and pressure. Bernoulli’s principle states that as the velocity of a fluid increases, the pressure exerted by the fluid decreases. This is evident in the design of an airplane wing.
 British scientist Robert Boyle examined the property of gases and developed Boyle’s law. This law states that as the volume of gas decreases, the pressure increases, provided the temperature does not change. It also says that as the volume of a gas increases, the pressure decreases. The pressure-temperature relationship states that as temperature increases, the pressure increases provided the volume does not change. For example, when your tire pressure is low in your car, it is usually due to a drop in temperature. When the temperature rises, so does the tire pressure.
British scientist Robert Boyle examined the property of gases and developed Boyle’s law. This law states that as the volume of gas decreases, the pressure increases, provided the temperature does not change. It also says that as the volume of a gas increases, the pressure decreases. The pressure-temperature relationship states that as temperature increases, the pressure increases provided the volume does not change. For example, when your tire pressure is low in your car, it is usually due to a drop in temperature. When the temperature rises, so does the tire pressure.
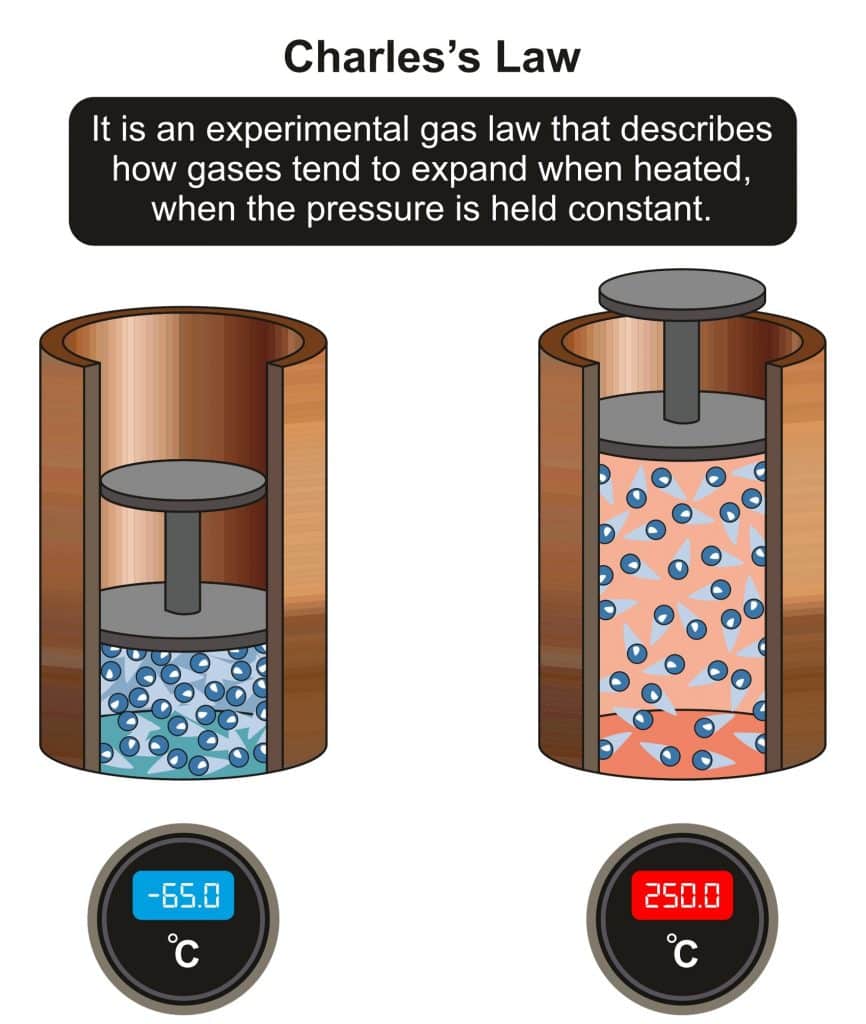
Jacques Charles was also a scientist who observed the properties of gases. Charles’s law states that as the temperature increases, the volume increases, provided the pressure remains constant. This can be seen in a hot air balloon. For example, a hot hair balloon rises because as the air inside is heated, the molecules gain energy and move farther apart, increasing the air volume inside the balloon.
Review:
- What is buoyancy?
- Explain Bernoulli’s principle.
- Explain the pressure-temperature relationship.
