Section 3: Strengths of Acids and Bases
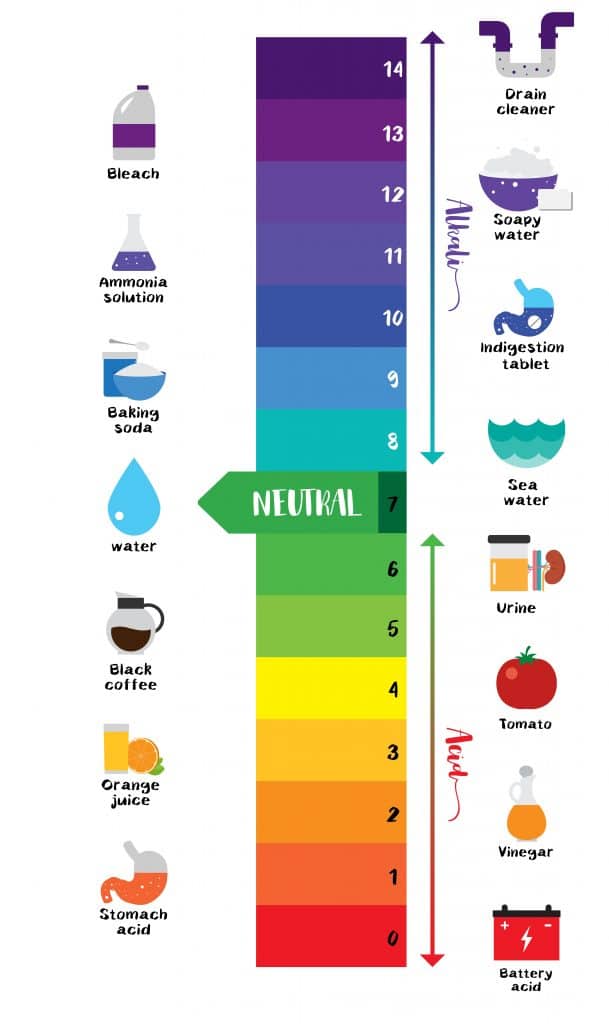
The pH scale Universal Indicator
The strength of an acid or base depends on how many acid or base particles dissociate into ions in water. A strong acid ionizes almost entirely in water, whereas a weak acid only partly ionizes in a solution. A strong base dissociates completely in a solution, whereas a weak base does not.
Strength and concentration are not the same things. Strength refers to how strong or weak something is. These terms refer to the ease at which an acid or base dissociates in a solution. When discussing concentration, the terms dilute and concentrated indicate the amount of acid or base in a solution. It is possible to have dilute solutions of strong acids and bases as well as concentrated solutions of weak acids and bases.
pH measures the concentration of H+ ions in a solution or how acidic or basic it is. A scale ranging from 0-14 is used to indicate the pH of a solution. The strength of the pH scale is determined by the concentration of hydrogen ions H+. The greater the concentration of H+, the lower the pH and the more acidic a solution is. Solutions with a pH lower than 7 are described as acidic, and the lower the value, the more acidic the solution is. Solutions with a pH greater than 7 are considered basic; the higher the pH, the more basic a solution is. A pH of precisely 7 indicates a neutral solution.

Review:
- What is the difference between strength and concentration?
- What does pH measure?
