Section 1: Types of Chemical Bonds
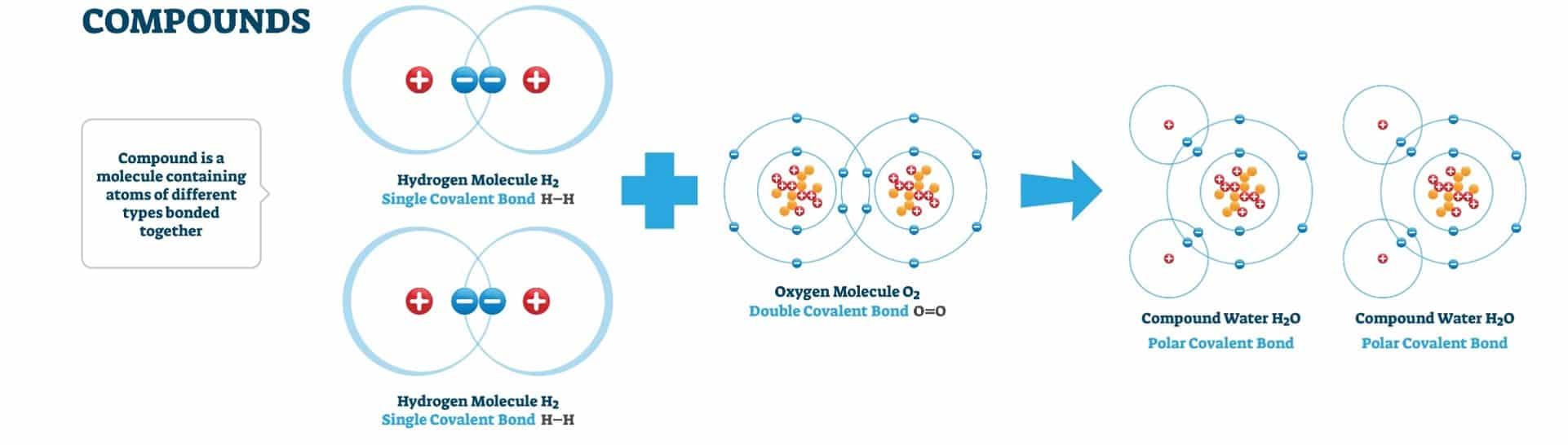 Each element can combine in many different ways to make millions of compounds. Scientists look at how and why elements react with each other to form compounds. A compound is a substance that is made of the combined atoms of two or more elements. For example, H20 combines two hydrogen atoms and one oxygen atom. A chemical formula tells us the exact number of atoms of each element a compound is made up of. When looking at a chemical formula, we look at the coefficient, subscript, and superscript to help us identify the compound. The coefficient represents the number of units of each of the substances. The subscript represents the number of atoms in a molecule of that particular element. The superscript represents the oxidation number or how many electrons have been gained or lost.
Each element can combine in many different ways to make millions of compounds. Scientists look at how and why elements react with each other to form compounds. A compound is a substance that is made of the combined atoms of two or more elements. For example, H20 combines two hydrogen atoms and one oxygen atom. A chemical formula tells us the exact number of atoms of each element a compound is made up of. When looking at a chemical formula, we look at the coefficient, subscript, and superscript to help us identify the compound. The coefficient represents the number of units of each of the substances. The subscript represents the number of atoms in a molecule of that particular element. The superscript represents the oxidation number or how many electrons have been gained or lost.
Atoms form compounds to become chemically stable. An atom is chemically stable when the outer energy level is complete. Atoms gain, share or lose an electron to become stable. An atom that has lost or gained an electron is called an ion. It is a charged particle with either fewer or more electrons than protons, resulting in a negative or positive charge. A chemical bond is a force that holds together the atoms in a substance to make compounds more stable. There are two kinds of bonds formed. One atom gives away its electrons in an ionic bond, and another accepts them. As a result, each has a full outer shell of electrons, making it neutral because the total ion charge is zero. In a covalent bond, the atoms sit together and share their electrons so that they both have full outer shells. For example, a water molecule consists of two hydrogen atoms and one oxygen atom. Each hydrogen atom shares its single electron with the one from the oxygen atom, forming covalent bonds. Some elements, like noble gases, do not give up their electrons, so they don’t usually react with other elements.

Review:
- In the compound H2O the 2 is a ________.
- What is an ion?
- Explain the difference between an ionic bond and a covalent bond.
