Section 2: Acids, Bases, and Salts
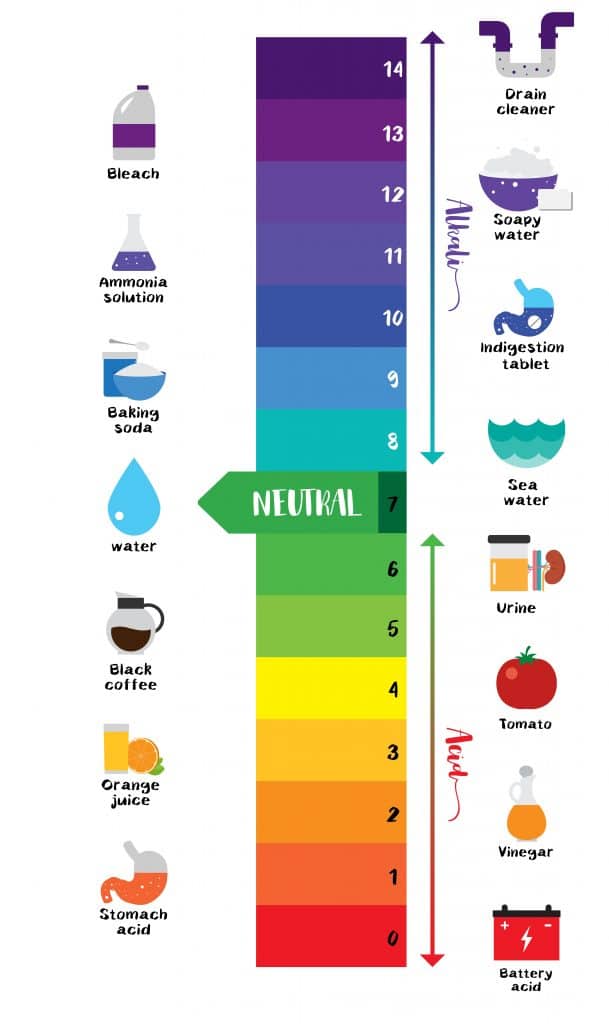
The pH scale Universal Indicator pH Color Chart diagram
Both acids and bases are found in everyday aspects of life. You have probably heard the term acidic when referring to fruits like lemons and oranges. An acid is a substance that produces hydrogen ions (H+) in a solution. The ability to produce these ions gives acids their distinct characteristics. All acids taste sour and are electrolytes, meaning they can conduct electricity in a solution. They are corrosive, which means they can be so strong they can eat away metals; acids react with indicators to produce a predictable color change. An indicator is a compound that changes colors to help determine whether something is an acid or a base. For example, litmus turns red when something is acidic and blue when something is basic.

Like acids, bases can be found around our homes in hand soaps, cleaning products, and other products. A base is a substance that produces hydroxide ions (OH-) in a solution and also accepts H+ from acids. All bases share specific characteristics. In an undissolved state, many bases are crystalline solids. In a solution, a base feels slippery. Bases also have a bitter taste, and strong bases are corrosive and can cause damage to the skin. When an acidic and basic solution are combined, neutralization occurs, forming water and salt. Neutralization is a chemical reaction between an acid and a base in a water solution. A salt is a compound formed when negative ions of an acid combine with positive ions from a base. (acid + base = salt + water)
Review:
- Identify three characteristics of acids.
- Identify three characteristics of bases.
- What is neutralization?
